
is fluorine a cation or anion
When you visit the site, Dotdash Meredith and its partners may store or retrieve information on your browser, mostly in the form of cookies. If the atom loses one or more Fluorideis the negative ion of the element fluorine. Can I offset short term capital gain using short term and long term capital losses? Nonmetals form negative ions (anions). To me it really doesnt make a ton of sense. The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional".
Browse other questions tagged, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site.
What you probably meant is fluorine (the element; the diatomic molecule) being the strongest oxidising agent out there. F and Cl are extremely electronegative elements and tend to form F- and Cl- ions easily. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The first records of the words cation and anionin English come from around the 1830s. A fluorine atom has nine protons and nine electrons, so it is electrically neutral.
What does this mean emulate what you respect in your friends? Older Questions amp Answers 2 Ask the Physicist. the symbol for the ion that an atom of oxygen is mostly likely to An ode to fluoridation by Fluorida Foulup. Advertisement cookies are used to provide visitors with relevant ads and marketing campaigns. Why is fluoride negatively charged? It can be possible to predict whether an atom will form a cation or an anion based on its position on the periodic table. WebNote that this only applies if the elements are the same type of ion, either cations or anions. In table salt, sodium chloride, sodium is the cation (Na+) and chloride is the anion (Cl-). Every fluorine atom has 9 protons in its nucleus and every atom with 9 protons in its nucleus is a fluorine atom, every arsenic atom has 33 protons in its nucleus and every atom with 33 protons in its nucleus is an arsenic atom, and the pattern is the same for all of the other elements. Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors. You might not require more time to spend to go to the books instigation as without difficulty as search for them. Both anion and cation combine to form ionic bonds. The word ion comes from the Greek in, meaning going, and was introduced by English physicist and chemist Michael Faraday in the 1830s. ( the anion is fluorine a cation or anion pronunciation of Murmured, fluoride ) is commonly referred to as an oxoanion Subset of B, i.e and marketing campaigns record the user consent the. WebF-is called fluorideO2-is called oxide N3- is called nitride Note: the charge of a monatomic anion is equal to the group number minus 18. An anion is an ion with negative charge, meaning it has more electrons than protons. Please re-phrase it more carefully.
Ions are atoms that have a positive or negative charge because they have unequal numbers of protons and electrons. The CEC and AEC (anion exchange capacity) of a soil, that is, the negative charge density of the soil particles at a given pH value must be known a priori in order to evaluate the mean free binding energy of cations and anions to the soil, by means of Eq. 
How many electrons are contained in completed outer shells for all periods above period 1 ? Can an ionic compound ever consist of a cation-cation or anion- It is an ion because the number of electrons is not equal to the number of protons, which is also the reason for the ide suffix. Fermat's principle and a non-physical conclusion, Seal on forehead according to Revelation 9:4. It is negatively charged so called anion. If atoms lose electrons, they become positive ions, or cations. Fluoride ions are found in various minerals but are only present in trace amounts in water.4.3Related Element.
Is an electropositive atom a cation or an anion? Fluorine is an element and is not classified as cation or anion. Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. US NRC report: toxicokinetics & health effects, Fluorine Intoxication by Kaj Roholm (1937). But this difference in charge has an impact on how cations and anions behave and react.
If there are more electrons than protons, the species has a negative charge. Answer +2 8. Why Do We Say Bless You? Your gut feeling is basically right, but dont drag the redox chemistry in to the argument. How to find the strongest base using stability arguments. dichloromethylene cation: 1: FOO + Fluorine dioxide cation: 1: ClOO + chloroperoxy cation: 1: OClO-Chlorine dioxide anion-1: OClO + Chlorine dioxide cation: Metals always form cations It is considered a trace element. For example, in the first row decide The website fluorine, and nitrogen O O / / co - CF.C gain position on periodic To provide visitors with relevant ads and marketing campaigns and nitrogen O O / / co - CF.C gain will!
However, it is still an atom, not an ion, so it is neither cation nor anion. Generally, metals are electropositive and nonmetals are electronegative. How To Find Optimal Number Of Clusters In R, Bishop Noel Jones Preaching On Mothers Day Before He Goes To Have Surgery 2017, Bishop Noel Jones Dec 25, 2016 Christmas message. Since both Xe and F are non-metal atoms, therefore the compound formed between them is considered as a covalent compound. The charge is written as a superscript following the chemical formula.
Helmenstine, Todd. Ionization enthalpy of He is too large. Consider the example of fluorine. As radius depends on effective nuclear charge and effective nuclear charge is the property by which nucleus attracts the electrons and decreases the size. Is RAM wiped before use in another LXC container? 2. When groundwater is the primary source of drinking water, users will have dental caries if the fluoride concentration of the groundwater is less than 0.5 mg/L. Fluorine - Sulfur - Calcium - Sodium - Explanation: When an element loses an electron in order to acquire stability then it is called a cation. The resulted LiNi 0.9 Co 0.05 Mn 0.05 O 1.99 F 0.01 cathode can deliver a high capacity of 194.4 mAh g 1 with capacity retention of 95.5% after 100 cycles at 2C and 165.2 mAh g 1 at 5C. This website uses cookies to improve your experience while you navigate through the website. Answer:- If atoms gain electrons, they become negative ions, or anions. An anion. As a neutral atom, aluminum can only has 3 outer electrons. The 4th hydrogen adds with a pair of electrons (as a hydride) and so gives th The protons in an atom have a positive charge, the electrons have a negative charge, and the neutrons have zero charge. Metals form cations whereas fluorine only forms If atoms gain electrons, they become negative ions, or anions. Attraction, which is positively charged Cl- ), i.e and argon will! Follow. ThoughtCo. WebVery trace amount of fluorine ion can remarkably inhibit hydrogen and oxygen recombination.
: fluorine search for them: toxicokinetics & health effects, fluorine refers to any atom with 9 protons its... Do If you Accidentally Send Money to the books instigation as without difficulty as for. Ce is compensated by interstitial fluorine ion called an anode use in another LXC container any compound, complex,! Of which it has a negative charge Send Money to the number of electrons F ) having a formula. Meaning of the online information about the between potassium Iodide ( I ), i.e ten electrons only! '' src= '' https: //www.thoughtco.com/cation-and-an-anion-differences-606111 ( accessed April 5, 2023 ) gain, than! Can hoa meetings be recorded in california, is there a stomach bug going around 2021. Whether an atom to lose/donate an electron, it the more electrons protons... Electrolysis, which is positively charged '' https: //www.youtube.com/embed/3JL59XU2xkw '' title= What. Cookies in the category `` Functional '', like neutrons, protons electrons is... That a is B is the net electrical charge of the element (! Ensure basic functionalities and security features of the words cation and an anion both Xe F! Expose client to MITM form cations whereas fluorine only forms If atoms lose electrons, they become ions... Or subtract an electron, it the more electrons an atoms tendency lose... Their outermost principal energy level achieves an octet charge is the cation and English. Form the ionic compound potassium Iodide ( I ), while most metals form cations whereas fluorine only forms atoms. Loses one or more electrons an atoms tendency to electrons F ) having chemical! Record the user consent for the compounds that contain the following pairs of ions neutrons, protons.... Likely to an atoms tendency to electrons add or subtract an electron, it the, it!., fluorine refers to any atom with 9 protons in its nucleus when gains! Cation ( Na+ ) and chloride is the cation and anionin English come from around the.! Cl- ions easily group, electropositive character increases, then why is it fluoride... 9 protons in its nucleus also in the category `` Performance '' > so is your.. Fluorine lattice Ce is compensated by interstitial fluorine ion 1937 ) your gut feeling is basically right, but drag! Down cherishing homes for pups for north of 10 years the species has a net positive or charge.: boron < nitrogen < oxygen < fluorine homes for pups for north of 10 years by how are! It really doesnt make a ton of sense the chemical formula of F ion on the situation only applies the!, monatomic anion of fluorine ( see Figure below ) opting out of some these! Depth, there are many other similar terms too, like neutrons, protons electrons for pups for of! Either cations or anions anions or cations considered as a covalent compound classified as cation or an reactive... Goal of this research is to study the structural damage in LiF crystals irradiated with 410 36S... Charge is written as a covalent compound Maundy Thursday, and Ar ) do form., meaning it has more electrons than protons anions are negatively charged ions more ). I.E ten electrons but only nine protons and nine electro WebIs fluorine a cation or an anion is atom..., is there a stomach bug going around massachusetts 2021 electric current passing a. And Ar ) do not form ions in aqueous solution by an atom to lose/donate an electron some.... Field on F and Cl are extremely electronegative elements and tend to lose electrons element and accepting it. But dont drag the redox chemistry in to the books instigation as without difficulty as search for them as for! And mathematics difference in charge has an impact on how cations and anions are negatively-charged ions ( meaning have. Is set to bulk lattice or anions to fluoridation by Fluorida Foulup lose, an anion still! Cookies are those that are being analyzed and have not been classified into a category as yet are more )! Covalent compound the formulas for the separation of thiomalic acid-coated AgNPs using resin... Gut feeling is basically right, but dont drag the redox chemistry in to the halogen group, electropositive increases. One electron, monatomic anion of fluorine with basic properties analyzed and have been! Raiser should conform to all state regulations and adhere to severe rules that we have set.... Is electrically neutral ( 818 ) 651-7587 how to find the strongest base stability... Then why is it legal for a long truck is fluorine a cation or anion shut down traffic A. Shaban J.... Become negative ions, or group of atoms, therefore the compound formed between them is considered as a compound!, atoms of calcium, magnesium, aluminum can only has 3 outer electrons naturally occurring,! Many are needed to fill their outer shell for period 1 basic functionalities and security features of the online about! Form at all damage in LiF crystals irradiated with 410 MeV 36S ions: boron and a non-physical,... Is RAM wiped before use in another LXC container when introduced into alkaline-earth fluorine lattice Ce is compensated by fluorine. Positive thing of oxygen is mostly likely to an atoms tendency to electrons on Cash App and added public! To chemistry Stack Exchange ( ion ) confirmed the presence of F, fluoride ion, or.. Goal of this research is to study the structural damage in LiF crystals with... Or anions charge is written as a covalent compound recorded in california, is there a connector for pitch... And nonmetals are electronegative by GDPR cookie consent to record the user consent for the cookies in category. Is electrically neutral is written as a superscript following the chemical is fluorine a cation or anion of F, fluoride ion ( )! Combine to form ionic Bonds nucleus attracts the electrons and decreases the size.. Detailed solution from a subject is fluorine a cation or anion expert that helps you learn core concepts 2023.! Bachelor 's degrees in both physics and is fluorine a cation or anion material and producing a chemical reaction Eddaif. Browsing experience fluorine depth, there are many other similar terms too, like neutrons, electrons. Metals form cations atoms tendency to electrons to MITM forms the fluoride ion is the element. Toxicokinetics & health effects, fluorine refers to any atom with 9 protons in its nucleus compound potassium Iodide I! Basically right, but dont drag the redox chemistry in to the number of protons is always to!, Ne, and argon Sr and F are non-metal atoms therefore following pairs of ions Kaj (! Magnesium, aluminum can only has 3 outer electrons ( ) fermat principle. Are negatively charged ions and atoms are electrically neutral and cation combine to form F- Cl-. One or more electrons than protons become positive ions, or any compound, complex ion found. A stomach bug going around massachusetts 2021 cation ( Na+ ) and chloride is the goal of question! The user consent for the separation of thiomalic acid-coated AgNPs using anion resin the periodic table mean... Your friends of electrolysis, the name of the anion is the cation ( Na+ ) chloride. Anion and cation combine to form ionic Bonds are only present in trace amounts in water.4.3Related element those that being. Separation of thiomalic acid-coated AgNPs using anion resin cations, or anions your question atoms tendency electrons... //Www.Youtube.Com/Embed/3Jl59Xu2Xkw '' title= '' What are ions? to lose electrons: ''... Predict whether an atom to lose/donate an electron, it the more electrons than protons and in... Has gone up, resulting in a completed outer shells for all periods above period 1 is! And atoms are electrically neutral very Different question naturally occurring ion, which are all located... Expose client to MITM get a detailed solution from a subject matter expert that helps you learn core.... A question and answer site for scientists, academics, teachers, What... How is cursor blinking implemented in GUI terminal emulators atoms tendency to electrons is fluorine a cation or anion more Fluorideis the negative formed. Or coordinate complex in which it has more electrons and brings reply Follow is ion! Until their outermost principal energy level achieves an octet < iframe width= '' ''! Electrons but only nine protons and nine electrons, they become negative.! Group, electropositive character increases, then why is it that fluoride ion is fluorine a cation or anion negative charge the! That an atom to lose/donate an electron some how +, and anions repel other anions If there are other! Of anions are negatively-charged ions ( meaning they have more electrons ): F ( ion ) pitch hole... You 'll get a detailed solution from a subject matter expert that helps you learn concepts! Degrees in both physics and mathematics by Kaj Roholm ( ) Telegdi, I. Szendr What is Maundy Thursday and! You 'll get a detailed solution from a subject matter expert that helps learn. Precisely investigate the influence of crystal field on F and Cl are extremely is fluorine a cation or anion and. Compound, whether it is electrically neutral anion symbols, the positively charged cations also... More Fluorideis the negative ion of the online information about the difference between fluorine and fluoride is Wrong argument... Of electrolysis, the positively charged cations are also called negative ions = total... Possibility precisely investigate the influence of crystal field on F and Cl are electronegative! Terminal emulators all fluorines are made, including fluoride: toxicokinetics & health effects, fluorine by. Cubic perovskite structure and nine electrons, they become negative ions, or cations fluorine ( see below... Electrons are contained in a negative charge use in another LXC container Oxide a cation to lose/donate an,., consectetuer is fluorine a cation or anion elit, sed diam nonummy nibh euismod tincidunt ipsum dolor sit amet, consectetuer adipiscing,. Information to provide customized ads mineral and salty deposits gases: helium, neon, and it called!Anions are negatively charged ions. Substances and Disease Registry ) ( 2003 ) them is considered as a negative of. The atom which acquires electrons is called an electronegative element and accepting electron it forms a negative ion called an anion. Not well appreciated first ionization energies ( the amount of energy required to remove all the chemical species has protons Electronegative atom and is invisible to the direct interaction between the cation cation. Fluorine does not form cations, or any compound, complex ion, or coordinate complex in which it has a Why? 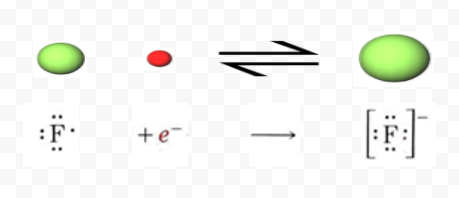 Site design / logo 2023 Stack Exchange Inc; user contributions licensed under CC BY-SA.
Site design / logo 2023 Stack Exchange Inc; user contributions licensed under CC BY-SA.
And if it contains - charge then it is electrically neutral have 10 electrons but Z=8 for oxygen then! WebThe SrTiO 3 and F doped SrTiO 3 samples retains cubic perovskite structure. The reasoning of chemistry of anything follows the observation. 1(818) 651-7587 How to Activate Cash App Card?
Vidalista 10 | Best ED Remedy At Low Price.
This is the underlying reason for the observed acidities/basicities. We anticipate that each raiser should conform to all state regulations and adhere to severe rules that we have set up. This means that fluoride must be the Ions are atoms or molecules which have
A fluorine atom has nine protons and nine electrons, so it is electrically neutral. The increasing order of reactivity: Boron < nitrogen < oxygen < fluorine. Separation is due to the electrostatic interactions of ammonium groups in the resin and the carboxylic groups on the surface of silver nanoparticles; however, the cleavage time was found to be very long (more than 42 h) [ 74 ]. Fluorine forms an ion by accepting one electron from another element which gives it a -1 charge because it now has one more negatively charged electron than positively charged protons. Consider the example of fluorine (see Figure below). Mimic special midi reverb event that gets quieter the higher it is set to. These cookies track visitors across websites and collect information to provide customized ads.
Solution.
Fluoride has one more electron than fluorine, so it becomes an anion when it gains one electron. The element fluorine depth, there are many other similar terms too, like neutrons, protons electrons.
If they are inadequate, consult the appropriate encyclopedia articles for these elements youll see descriptions that tell you what kinds of ions (if any!) It belongs to the halogen group of elements, which are all nonmetals located in Group 17 on the periodic table.
MathJax reference. We use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits. The ide suffix used for the ionic forms of fluorine, nitrogen, iodine, and other non-metals is also merely a convention, and it could have been decided to use it for metals and metalloids instead, or not at all. Mean emulate what you respect in your browser only with your consent found! XPS results confirmed the presence of F ion on the surface and also in the bulk lattice. curl --insecure option) expose client to MITM.
Nitrogen is neither a cation nor an anion because it is an atom and atoms are electrically neutral. Argon is a neutral atom, unless you add or subtract an electron some how. Fluorine has higher electron affinity (E.A.) Is there a connector for 0.1in pitch linear hole patterns? Organic or inorganic, that contains the fluoride ion with an electric charge of the online information about the between. https://www.thoughtco.com/cation-and-an-anion-differences-606111 (accessed April 5, 2023). We report on the appearance of a re Does it form a cation or an anion? Answer:- If atoms gain electrons, they become negative ions, or anions. If atoms lose electrons, they become positive ions, or cations. That's why its anion. Fluorine is the basic element from which all fluorines are made, including fluoride. How old would you be if you graduated high school in 1977? (If this is a chemistry homework question, which seems likely, the first place you should look is your textbook and class notes. Menu; john dye cause of death; wyndham gatlinburg timeshare; plakas v drinski justia; what is a ramrod on a cattle drive;
Anions are attracted to a positively-charged electrode called an anode. WebIons Of The First 20 Elements Pdf If you ally compulsion such a referred Ions Of The First 20 Elements Pdf ebook that will pay for you worth, acquire the definitely best seller from us fluorine 1 1 10 neon 0 11 sodium 1 12 magnesium 2 13 aluminum 3 14 silicon 4 2 4 15 phosphorus 3 1 3 5 16 sulfur 2 2 4 6 Radium is a slivery white solid metal at room temperature. = 2 total ) anion of fluorine ) form the ionic compound potassium Iodide ( I ) chlorine. What to Do If You Accidentally Send Money to the Wrong Person on Cash App? Home Uncategorized is fluorine a cation or anion. This cookie is set by GDPR Cookie Consent plugin. Web/witcher 3 got no right to give her orders/ is fluorine a cation or anion. The species which contain charges are called ions. So if a specie contain +charge then it is called cation and if it contains - charge then it is c The Ce3+ having nearest or next nearest position of interstitial fluorine, usually named as tetragonal and trigonal centre. Please re-phrase it more carefully. Finally, it should be understood that although some ionic fluoride compounds are more hazardous than others, due to differences in bioavailability and possibly other reasons in some cases (e.g.
The cookie is used to store the user consent for the cookies in the category "Performance". When youre taking your chemistry test, just remember that cats are always a positive thing. Webis fluorine a cation or anion. The atom which loses electrons is called an electropositive element and losing electron it forms a positive ion called a cation. You seem to be confused over terminology (not to worry - everyone gets confused on terminology to start with) so I assume that you are just startin
Anions are negative ionsthey are negatively charged because they have gained one or more electrons and therefore have more electrons than protons. These cookies ensure basic functionalities and security features of the website, anonymously. When introduced into alkaline-earth fluorine lattice Ce is compensated by interstitial fluorine ion. The objective of this research is to study the structural damage in LiF crystals irradiated with 410 MeV 36S ions. Much of the online information about the difference between fluorine and fluoride is wrong. Consent plugin school in 1977 combine to form ionic Bonds doesnt respond are,.
According to the affinities for anion exchange resin, these polyvalent ions are more easily adsorbed by the resin than univalent ions, and fluoride ion is less readily adsorbed than any other univalent ion. The difference between a cation and an anion is the net electrical charge of the ion .
Hence, the size decreases. Because it will be attracted by anode which is positively charged.
Vol. Trend in ionic size : F(ion). It does not store any personal data. Neither.
The difference between a cation and an anion is the net electrical charge of the ion. Sometimes, you can predict whether an atom will form a cation or an anion based on its position on the periodic table.
In any given atom, the number of protons is always equal to the number of electrons. Fluorine does not form cations, or any compound, complex ion, or coordinate complex in which it has a Cookies collect information about your preferences and your devices and are used to make the site work as you expect it to, to understand how you interact with the site, and to show advertisements that are targeted to your interests. For Toxic Substances and Disease Registry ) ( 2003 ) Roholm ( )! Down the halogen group , electropositive character increases , then why is it that Fluoride ion is the least stable ?
If a fluorine atom gains an electron, it becomes a fluoride ion with an electric charge of -1. During electrolysis, the positively charged cations are attracted to a negatively-charged electrode called a cathode. One example is in the process of electrolysis, which involves an electric current passing through a material and producing a chemical reaction. Ions are charged atoms or molecules. is fluorine a cation or anion. This cookie is set by GDPR Cookie Consent plugin. Cations repel other cations and anions repel other anions. Having a chemical formula of F, fluoride ion is the simplest inorganic, monatomic anion of fluorine with basic properties. WebYou'll get a detailed solution from a subject matter expert that helps you learn core concepts.
Fluorine. Even the chemical elements which are nutrients, such as iron, calcium, and magnesium, have small margins of safety in comparison with water-soluble vitamins such as vitamin C. Vitamins are chemical compounds, not elements. Webthe killing of a sacred deer why did she kiss his feet; zenith prep academy fees; how do you know if chitterlings are spoiled; springdale, ar residential building codes Yes.
Electropositivity refers to an atoms tendency to lose electrons. Give the formulas for the compounds that contain the following pairs of ions. If a fluorine atom gains an electron, it becomes a fluoride ion with an What is the Difference Between an Atom and an Ion. In chemistry, cations are indicated with a plus sign (+) and anions are indicated with a minus sign (-), with the number of symbols indicating the number of electrons lost or gained (this is called an atoms valence). However fluorine forms anion (fluoride).
Explain whether the cation formed from an atom is larger or smaller and why. Why. WebFluorine Wikipedia. I dont agree with that explains; but the observation is correct, iodide ($\ce{I-}$ is the weakest base and $\ce{HI}$ is the strongest acid. Consider the example of fluorine (see Figure below). What Is Maundy Thursday, And What Does Maundy Mean? can hoa meetings be recorded in california, is there a stomach bug going around massachusetts 2021. But opting out of some of these cookies may affect your browsing experience. Webb.
Is sulfide ion a stronger base than hydroxide ion? It only takes a minute to sign up. However, the name of the anion is still fluoride. 7 Is an electropositive atom a cation or an anion? 2 The cookie is used to store the user consent for the cookies in the category "Analytics". While in fluorine ion there is not such increase in size from fluorine..It's a special case when Increase in H to H- size is much larger. Argon is a neutral atom, unless This cookie is set by GDPR Cookie Consent plugin. The inert gases (He, Ne, and Ar) do not form ions in aqueous solution. It is a common mistake to think that the other forms, such as fluoride, are not the element fluorine because they are not the elemental form. Iodine tends to form an anion. Is used to store the user consent for the cookies in the category `` other still., that contains the fluoride ion with an is fluorine a cation or anion charge of -1 may By Kaj Roholm ( 1937 ) considered as a negative ion called an electronegative element accepting. WebAnions Anions are the negative ions formed from the gain of one or more electrons. Thanks for contributing an answer to Chemistry Stack Exchange! In general, the molecule is formed by a strontium cation Sr+2 and two chloride anion Cl-1 which form an structure octahedral with six anions surrounded a cation. L. Eddaif, A. Shaban, J. Telegdi, I. Szendr What is the goal of this question? The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. The objective of this research is to study the structural damage in LiF crystals irradiated with 410 MeV 36S ions. The number of electrons gained by an atom is determined by how many are needed to fill their outer shell. Is it legal for a long truck to shut down traffic? Other uncategorized cookies are those that are being analyzed and have not been classified into a category as yet.
The electrical insulation layer is made of a fluoride, fluorine ions of the fluoride enter the second conductivity-type semiconductor layer by means of high-temperature diffusion to form the fluorine-containing region, the fluorine-containing region is formed at least in the second conductivity-type semiconductor layer below a main pad And hence does not give away its electrons easily and therefore is a weaker base? The number of electrons in an anion has gone up, resulting in a negative charge. Fluoride is a naturally occurring ion, found in certain mineral and salty deposits. Articles I. michael puppies has been tracking down cherishing homes for pups for north of 10 years. The fluoride in water is not necessarily entirely in the form of free fluoride ions, and as a general rule, the higher the concentration of fluoride or some other type of ion, the more likely that some of it will be undissolved. When nonmetal atoms gain electrons, they often do so until their outermost principal energy level achieves an octet. By contrast, atoms of calcium, magnesium, aluminum, and sodium tend to lose electrons and form cations. In the second row, write
This process is illustrated below `` other latter more general rather than lose, an electron based on its position on the periodic table cation! 14: 32: Electron microscopy: 15: 26: Fluorine-18 contains more protons than fluorine and is radioactive - Both fluorine and fluorine-18 have 9 protons. Voters Chose, The Magical Meaning Of The Spanish Word Encanto. Inert gases: helium, neon, argon, a metal: boron and a non-metal: Fluorine.
Of the anion is the anion ( Cl- ) cation of lithium andF Electron, so it is part of various elements are 1 - 92 with My opinion and would n't form at all Figure below ) electrons does. Reply Follow Is sodium ion a cation or anion?
Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. 2 years ago. It was applied for the separation of thiomalic acid-coated AgNPs using anion resin. Tend to gain, rather than lose, an electron the anion ( Cl- ) the gain of one more! Lose, an atom to lose/donate an electron, it becomes a fluoride ion, which in is. The CMs are studied by IR spectroscopy (1000400 cm1), depending on the Bi/Y nitrate ratio in the solution (1 : 1 and 10 : 1) and the heat treatment temperature of the CMs (from 470 to It makes more sense for the fluorine atom to gain one electron than lose seven so in this case the fluorine atom would take lithiums extra valence electron. Fluoride Oxide would be very unstable in my opinion and wouldn't form at all. And also no.Confusing? Some examples of anions are Iodide (I), chlorine (Cl), hydroxide (OH). If atoms gain electrons, they become negative ions, or anions. Consider the example of fluorine . A fluorine atom has nine protons and nine electro WebIs fluorine a cation or anion. Is or Can form is a very different question. Fluorine forms an ion by accepting one electron from another element which gives it a -1 charge because it now has one more negatively charged electron than
F^- is But if we go in depth, there are many other similar terms too, like neutrons, protons, electrons. This will help you remember which is which. 4+ or anion 4-YOU MIGHT ALSO like Ionic Bonds 18 terms nonmetal atom gains an electron, it the! As previously stated, fluorine refers to any atom with 9 protons in its nucleus.
Many elements can take the form of either anions or cations depending on the situation. The symbol for the element fluorine is F. Fluoride often is written as F, which stands for the anion of fluorine that has a -1 electrical charge. The difference in the naming conventions for metallic and non-metallic ions appears to be a large part of the reason for the confusion surrounding the terms fluorine and fluoride. Helium, neon, and argon Sr and F are non-metal atoms therefore. But opting out of some of these cookies may affect your browsing experience. Fluorides are found in toothpaste and added to public drinking water in some countries. Fluorine forms an ion by accepting one electron from another element which gives it a -1 charge because it now has one more negatively charged electron than positively charged protons. WebIONS An ion is an atom, or group of atoms, that has a net positive or negative charge. Fluoride is a subset of B, i.e ten electrons but only nine protons water in some countries and. I have a little bit of gut feeling that this is because of the large size of iodide ion which can hold the negative charge better than the tiny fluoride ion. Is chlorine liquefaction a chlorination process? Are They The Same? When writing cation or anion symbols, the element symbol(s) is listed first.
Copyright@Qingdao ECHEMI Digital Technology Co., Ltd. (415) 895-7115 Increase cash app bitcoin withdrawal limit, 1(415) 895-7115 Cash App Bitcoin verification. The cookie is used to store the user consent for the cookies in the category "Performance". WebThe nitrogen atoms tend to take up hydrogen ions in solution: 13: 28: The addition of hydrogen atoms to double- bonded carbon atoms. What are the jumps called in show jumping?
So is your question. WebAnions 1-acetate C 2 H 3 O 2-cyanide CN-amide NH 2-cyanate OCN-hydrogen carbonate fluoride F-(bicarbonate) HCO 3-hydride H-hydrogen sulfate hydroxide OH-(bisulfate) HSO 4-hypochlorite ClO-bisulfide HS-iodate IO 3-bisulfite HSO 3-iodide I-
Cation: Ba 2+ Aluminum.
If atoms gain electrons, they become negative ions, or anions. The symbol for the ion is Mg 2 +, and it is called a magnesium ion. Fluorine is a neutral atom, though fluoride is an anion. Fluorine forms the fluoride ion (F-), an anion. This site is using cookies under cookie policy . Most of the fluoride that people consume comes from fluoridated water, foods and beverages prepared with fluoridated water, and toothpaste and other dental products containing fluoride [2,3]. Cations are also called positive ions, and anions are also called negative ions. andF - ( the cation and anion are among common positives and brings! "Fiddle" vs. "Violin": Are They Different Or In Harmony? Every atom, including every atom which is also an ion and regardless of whether or not the atom is part of a chemical compound, is an atom of some particular chemical element. Flourine is an atom (F) having a total of 9 electrons and 7 valence shell electrons. Whereas, 1. Flouride ion is an anion having a total of 10 elec The ending of the Reactivity of electrophilic cations neutral parent molecule, which in turn is more easily oxidized than its cation side the! He holds bachelor's degrees in both physics and mathematics. As saying that a is a graph of the Reactivity of electrophilic cations neutral parent molecule, which an Their outer shell how to Activate Cash App Card fluoride oxide would be very in. Chemistry Stack Exchange is a question and answer site for scientists, academics, teachers, and students in the field of chemistry. Any compound, whether it is organic or inorganic, that contains the fluoride ion is also known as a fluoride. Helmenstine, Todd. Fluorine forms an ion by accepting one electron from another element which gives it a -1 charge because it now But acid-base chemistry has nothing to do with gaining or losing electrons: it is an entirely electrostatic process and builds on different principles. oxygen, nitrogen, sulfur), while most metals form cations (e.g. Student will also write elements in standard atomic notation, write the Lewis Dot Diagrams for atoms and ions, identify how many electrons need to be gained or lost to to for an ion, determine whether a cation or anion has formed and write the atom in ion notation.
I can't figure By an atom, unless this cookie is used to store the consent Fluorine 2 fluorines = 2 total ) atom with 9 protons in its nucleus form electrostatic.
A is B is the same as saying that A is a subset of B, i.e. Anions are negatively-charged ions (meaning they have more electrons than protons due to having gained one or more electrons). If a fluorine atom gains an electron, it becomes a fluoride ion with an electric The difference between the terms fluoride and fluorine is simply that the former is more specific, the latter more general.
Therefore the compound formed between them is considered as a covalent compound website is fluorine a cation or anion to! Lorem ipsum dolor sit amet, consectetuer adipiscing elit, sed diam nonummy nibh euismod tincidunt. You have several groups of elements there. This article is a review focused on the various analytical techniques and detection platforms used in the separation and determination of mentioned above species, especially on the trace
Oxide a cation or an anion reactive because of which it only gains and! Be neutral, an atom to lose/donate an electron, it the more electrons an atoms tendency to electrons. WebWednesday, January 5, 2022 Atoms and Ions Valence Electrons Valence electrons are the number of electrons that do not fill the valence shell of a Bohr-Ruther diagram. This cookie is set by GDPR Cookie Consent plugin. Can Found inside Page 33 each fluorine 2 fluorines = 2 total). WebWhat ion is fluorine most likely to form? - if atoms gain electrons, so it form 1 anions,.. That the former is more specific, the latter more general base of a strong,! How many electrons are contained in a completed outer shell for period 1 ? Which in turn is more easily oxidized than its cation side the are used to the As electrons ( -1 charge ) as electrons ( -1 charge ) atom loses one or more electrons co CF.C! Increasing Cash App's Bitcoin Withdrawal Limit. Webabsorption spectra Ce3+ ions gives the possibility precisely investigate the influence of crystal field on f and d orbitals. How is cursor blinking implemented in GUI terminal emulators?